Aging is an inevitable process that eventually weakens the muscles and immune system. The hair thins out, and memory dwindles. Many people also worry about the effect of old age on the healing effects on our body’s stem cells. A popular theory has it that as you age, stem cells become too old to use for regenerative orthopedic treatments. Some say you should instead use young stem cells from umbilical cord or amniotic fluid. However, the truth about this lies in learning about the relationship between stem cells and aging.
The Effect of Aging on the Stem Cells
Your body hosts millions of stem cells, which are the repairmen of many tissues. This means that wiping out the stem cells in the bone marrow can be life-threatening. It could result in death in a few weeks or months.
Traditionally, experts have believed that the health and number of stem cells decline with age. However, research shows little relationship between stem cells in the bone marrow and age.
What is the Age Limit?
A study involving more than 800 patients of different ages with knee arthritis sought to establish whether there is an age limit to bone marrow concentrate (BMC) treatments, where the body’s stem cells come from. These patients had their own concentrated bone marrow cells injected into their joints. The study found no relationship between age and the outcome as both young and old patients exhibited the same results.
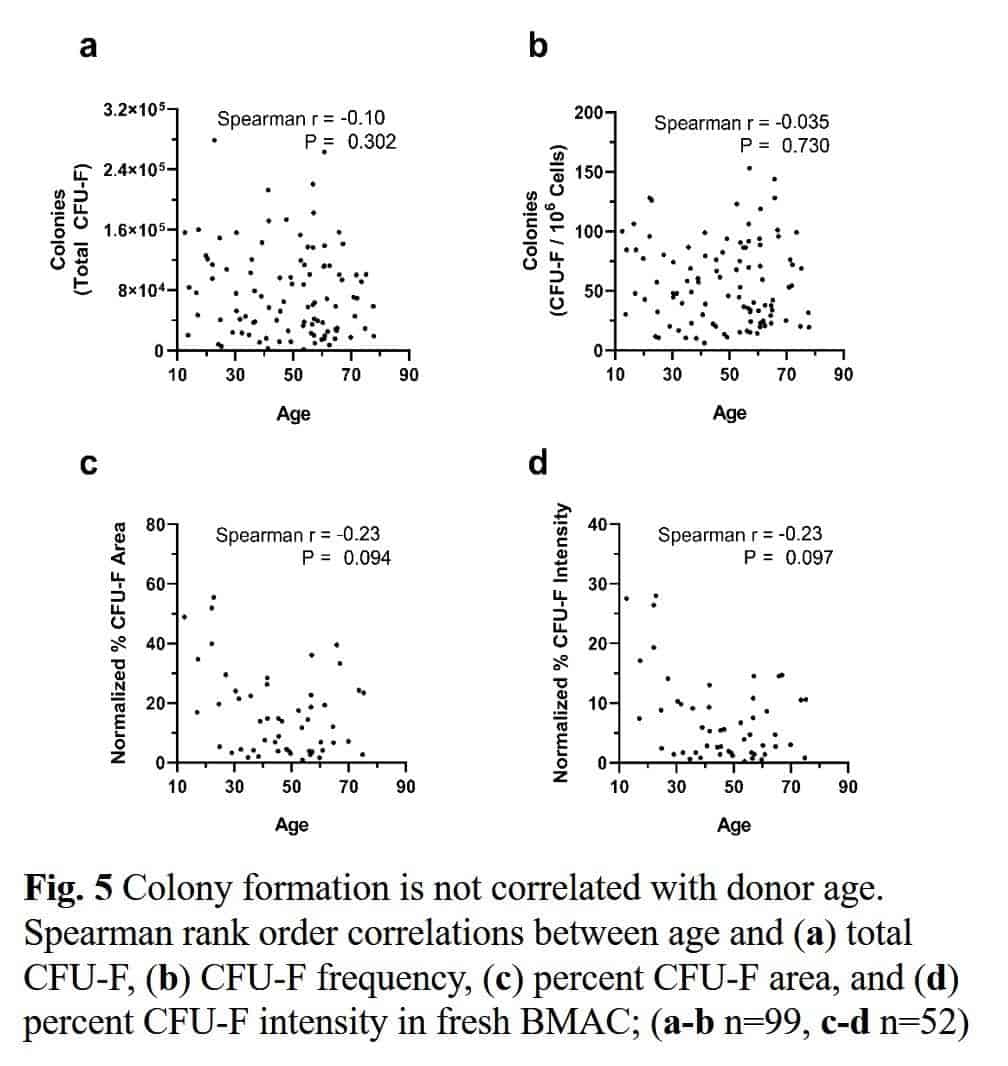
In a different study, the researchers established that one fact that contributes to poorer outcomes was too low a dose of the total bone marrow cells. However, clinicians can easily correct this by using a better process in drawing and processing the bone marrow derived cells. This means that older cells don’t hamper the chances of success. They only need to have a certain concentration level. Several other studies have also established this relationship between the outcome of cellular procedures and the cell dose.
What About “Young” Stem Cells?
Many “stem cell clinics” propose that stem cells lose their functionality with age and that you need younger cells from umbilical cords or amniotic fluid. This is a sales pitch they use to sell their therapies. Their claims are not factual as amniotic fluid and umbilical cord stem cell products don’t contain live and viable stem cells, as confirmed by various reputable labs.
The diagram below shows results from a joint study by Regenexx and a University lab. The results are from a stem cell test referred to as a CFU-f. Here, the stem cells show up as purple dots in groups or colonies. They represent the stem cells derived from bone marrow samples of patients in the ages of 50s to 70s. Their tests came back showing a high number of cells.
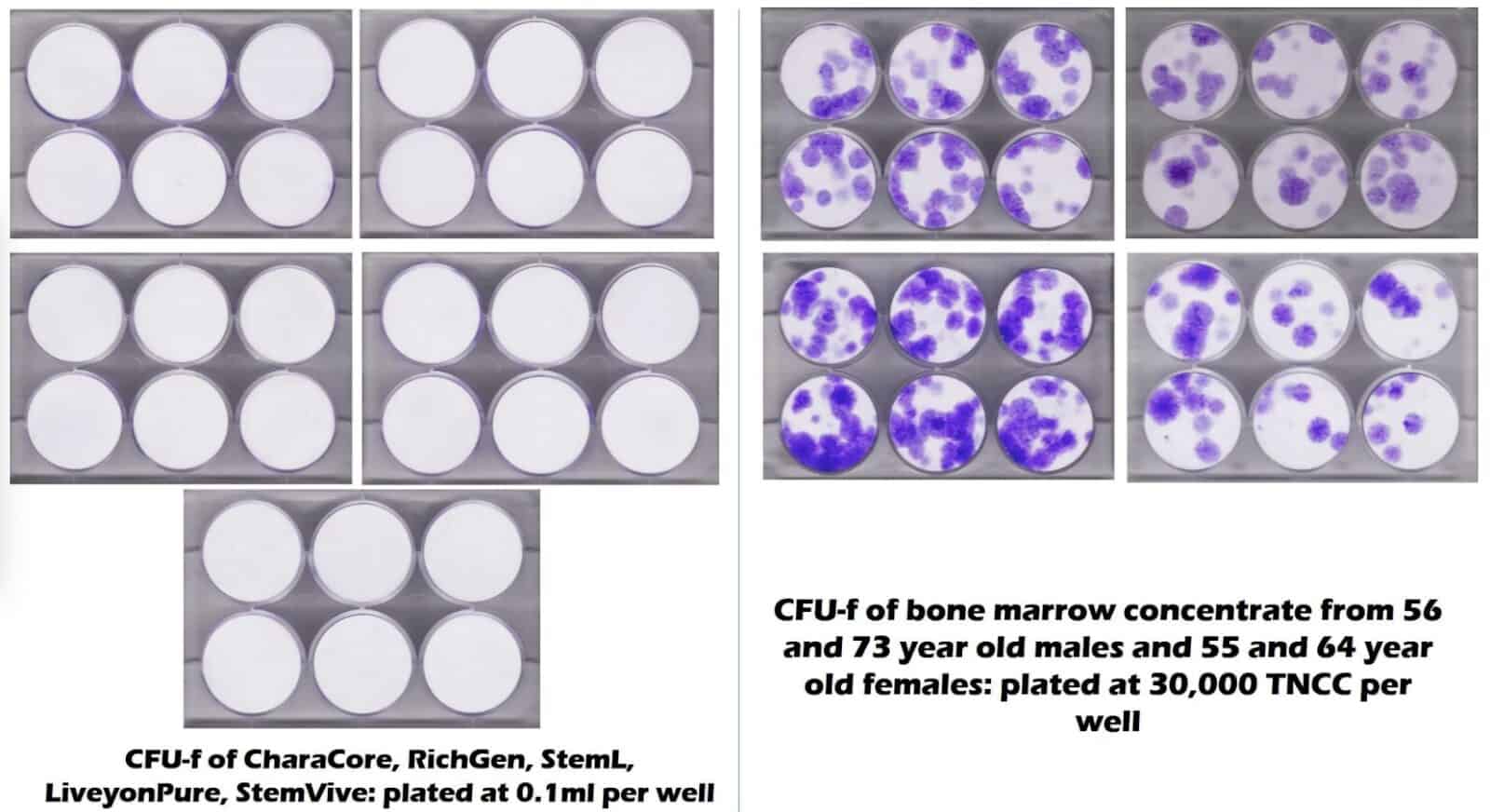
In the diagram with nothing but white circles, no cells exist. These represent five commonly used umbilical cord “stem cell” products. They are five wells of six white circles in a plate, with each plate being for each product.
Without a doubt, the sales pitch based on young stem cells is to entice you into stem cell therapy. Studies have proved that your own bone marrow contains viable stem cells even in old age.
The Other Limitations of Umbilical Cord Cells
Recently, masses of patients became critically ill because of polluted umbilical cord “stem cells.” Remember that the FDA does not approve those products, neither are they monitored like regular drugs. As such, contamination is a very real risk.
While aging slightly impacts stem cells, you still have many live and viable cells you can use. Before you consider the umbilical cord “stem cell” therapies, talk to one of our regenerative orthopedic physicians at Regenexx Tampa Bay.
References:
(1) Lee-Six, H., Øbro, N. F., Shepherd, M. S., Grossmann, S., Dawson, K., Belmonte, M. Campbell, P. J. Population dynamics of normal human blood inferred from somatic mutations. Nature 561, pages 473–478, 2018. doi:10.1038/s41586-018-0497-0
(2) Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells – current trends and future prospective. Biosci Rep. 2015;35(2):e00191. Published 2015 Apr 28. doi:10.1042/BSR20150025
(3) Green DE, Rubin CT. Consequences of irradiation on bone and marrow phenotypes, and its relation to disruption of hematopoietic precursors. Bone. 2014;63:87–94. doi:10.1016/j.bone.2014.02.018
(4) Ahmed AS, Sheng MH, Wasnik S, Baylink DJ, Lau KW. Effect of aging on stem cells. World J Exp Med. 2017;7(1):1–10. Published 2017 Feb 20. doi:10.5493/wjem.v7.i1.1
(5) Centeno C, Pitts J, Al-Sayegh H, Freeman M. Efficacy of autologous bone marrow concentrate for knee osteoarthritis with and without adipose graft. Biomed Res Int. 2014;2014:370621. doi:10.1155/2014/370621
(6) Centeno CJ, Al-Sayegh H, Bashir J, Goodyear S, Freeman MD. A dose response analysis of a specific bone marrow concentrate treatment protocol for knee osteoarthritis. BMC Musculoskelet Disord. 2015;16:258. Published 2015 Sep 18. doi:10.1186/s12891-015-0714-z
(7) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(8) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(9) Panero, A, Hirahara, A., Andersen, W, Rothenberg J, Fierro, F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine, 2019 47(5), 1230–1235. https://doi.org/10.1177/0363546519829034
(10) US Food and Drug Administration. “FDA sends warning to company for marketing dangerous unapproved stem cell products that put patients at risk and puts other stem cell firms, providers on notice” 12 August 2019, https://www.fda.gov/news-events/press-announcements/fda-sends-warning-company-marketing-dangerous-unapproved-stem-cell-products-put-patients-risk-and .
(11) US Food and Drug Administration. “Tissue Establishment Registration” 11 August 2019, https://www.fda.gov/vaccines-blood-biologics/biologics-establishment-registration/tissue-establishment-registration.
(12) Homma Y, Zimmermann G, Hernigou P. Cellular therapies for the treatment of non-union: the past, present and future. Injury. 2013 Jan;44 Suppl 1:S46-9. doi: 10.1016/S0020-1383(13)70011-1.
(13) Pettine KA, Suzuki RK, Sand TT, Murphy MB. Autologous bone marrow concentrate intradiscal injection for the treatment of degenerative disc disease with three-year follow-up. Int Orthop. 2017 Oct;41(10):2097-2103. doi: 10.1007/s00264-017-3560-9.